
Clean in Place (CIP): The Complete Guide
.webp)
The Complete Guide to Clean-in-Place (CIP) Process Types, Chemicals, & Equipment
I grew up on the floor of my grandfather's liquid manufacturing plant, and I've since analyzed thousands of CIP cycles across six continents. I've seen what works, what doesn't, and where the real inefficiencies hide.
So why am I writing this CIP guide? My company, Laminar, is the most globally deployed CIP optimization solution in the world — you'll find our sensors and ML models in AB InBev, Coca-Cola, and Unilever facilities across six continents.
This is the most comprehensive clean-in-place primer available — consider it your CIP 101.
Key Takeaways
This is an in-depth look into clean-in-place processes , chemicals, and equipment. Use the Table of Contents on the left to navigate quickly to certain sections. Otherwise, read on!
Here's what's covered below:
- CIP (clean-in-place): cleans equipment without disassembly using water, chemicals, and heat
- CIP processes: range from 1-step (hot water) to 7-step (full chemical + sanitizer cycles)
- Three main CIP chemicals: caustic (removes organics), acid (removes scale), sanitizer (kills microbes)
- CIP systems: Single-Use (Type 1) CIP skids use fresh chemicals each cycle; Reuse (Type II) recirculates and reclaims
- CIP frequency: varies by industry: 24-48 hours (dairy) to weekly (sauces, flavors)
What is Clean in Place (CIP)?
Clean-in-place (CIP) is a method of cleaning interior surfaces of pipes, vessels, tanks, and equipment without disassembly, using water, chemicals, heat, and flow to remove product residue and contaminants. Clean-in-place methods are used in liquid manufacturing and production facilities.
When is Clean-in-Place Triggered or Used?
The CIP cleaning process is done when the production line shifts from one product to another product in cases where the two products are not compatible. To ensure recontamination between the two products, a CIP is done to clean of equipment. Incompatible products might be going from a pungent flavor product to a non-pungent product or one with a specific allergen to one without.
In cases of compatible products like from a diet soda to regular soda, product lines may only require a product push or product changeover step to meet the required level of quality for production.
The types of CIP or changeovers needed to be run when switching between two products is determined by a changeover matrix. The complexity of the changeover matrix increases with increasing SKUs produced on a line and is an area of optimization for line efficiency.
CIP Cleaning Frequency by Industry
Clean-in-place procedures are also required on routine intervals after a set amount of production time. This is typically to disinfect and prevent microbial and contamination build up. In the dairy industry, this interval might be 24 to 48 hours. In beverages, this might be every 1 to 3 days. In sauces, this might be multiple days to a week. Cycle frequency can vary heavily from industry to industry.
Clean-in-Place vs. Clean out of Place (CIP vs COP)
What is Clean out of Place (COP)
Clean out of place is a method of taking equipment apart and cleaning it in a cleaning machine (like a dishwasher) or by hand. These are often fittings, joints, and specific components that are hard for CIP. In contrast, Clean in place is for equipment that does not need to be taken apart.
For example, unlike smaller parts, a 10,000-gallon tank or pipe infrastructure can't be disassembled and taken to a cleaning station. Instead, it's cleaned from a CIP skid that sends water and chemical solution into it to clean it.
The CIP + COP Cleaning Combo
In many facilities, CIP (clean-in-place) and COP (clean-out-of-place) both occur. In a line that does both CIP and COP and requires cleaning, the operator first disassembles the components on the line that requires COP and preps the line for CIP. The line can then undergo CIP and its disassembled components can undergo COP at the same time. After both CIP and COP is complete, the line can be reassembled to begin production.
CIP Cleaning Bottlenecks
This is where cleaning bottlenecks often hide. When CIP and COP are both done, one process is usually holding up the other. The key is understanding the full sequence: What's the CIP process? What's the COP process? How long does each take? And which one is the bottleneck preventing the line from getting back to production?
Facilities that map this out in their SOPs can often find significant time savings just by identifying which cleaning step is driving downtime. Additionally, by properly tracking the disassembly and setup of the equipment for CIP and reassembly of the equipment post CIP, substantial hidden downtime savings opportunities can also be revealed.

CIP Chemicals: Caustic, Acid, and Sanitizer Explained
The Three Main CIP Chemicals and Cleaning Solutions
CIP chemistry comes down to three workhorses: caustic, acid, and sanitizer. Each does a different job, and understanding what they're actually doing inside your equipment matters for optimization.
- Caustic (NaOH): High pH, breaks down fats/proteins; usually run hot
- Acid (Nitric Acid, Phosphoric, Citric Acid): Removes soils as well as mineral scale built up from high pH during the caustic wash.
- Sanitizer Detergent (PAA): Breaks down to water + vinegar; food-safe final kill step
Here's a closer look at each:
Caustic (NaOH — Sodium Hydroxide)
Caustic is your heavy lifter. It's a high-pH solution (typically 1-4% concentration) that breaks down organic soils—fats, proteins, starches, oils. If something is sticky, greasy, or baked on, caustic is what removes it.
Caustic is almost always run hot, usually between 65-80°C. The heat matters: it increases cleaning action and helps dissolve soils that would resist a cold wash. This is why "hot caustic" is standard in food and beverage CIP.
The tradeoff: caustic has a very high pH, which over time causes calcium carbonate buildup and mineral scale on pipe walls. This is why facilities running caustic-only CIPs eventually need an acid step to descale.
Acid (Nitric, Phosphoric, or Citric Acid)
Acid serves two purposes. First, it removes mineral deposits and scale—the buildup that accumulates from repeated caustic washes and from minerals in the water and product itself. Second, it handles soils that caustic can't touch, particularly mineral-based residues.
The type of acid matters:
- Nitric acid is the most common in food and beverage—aggressive on scale, widely available
- Phosphoric acid is gentler and often used where nitric is too harsh
- Citric acid shows up in facilities looking for a more environmentally friendly option, though it's less effective on heavy scale
Acid is typically run at lower concentrations than caustic (0.5-1.5%) and can be run at ambient or warm temperatures depending on the application.
Sanitizer Detergent (PAA — Peracetic Acid)
PAA is your final kill step used more in food and pharma and less in beverage production. It's a broad-spectrum antimicrobial that eliminates bacteria, yeast, mold, and viruses that survive the wash steps. The key advantage: it breaks down into water and acetic acid (vinegar), making it food-safe with no rinse required in many applications.
PAA is typically run cold and at low concentrations (100-200 ppm). It's fast-acting—contact times are usually just a few minutes.
One note: some facilities use hot water as a sanitizer step instead of PAA, relying on temperature alone (82°C+) to kill microbes. This works but uses significantly more energy.
CIP Chemical Handling, Dosing, and Concentration
CIP chemicals arrive at full concentration — caustic might come in at 50% NaOH, for example — and are stored in IBC tanks or drums. From there, they're dosed into the CIP skid's solution tank and diluted to working concentration, typically around 1-4%.
Dosing is usually controlled by conductivity. The CIP system monitors conductivity in the solution tank or return loop and doses chemical to maintain a target setpoint (commonly 30-50 mS/cm for caustic). When conductivity drops below setpoint, more chemical is added.
CIP Chemical Dosing: The Inline Dosing Problem
Some facilities use inline dosing—adding chemicals directly into the flow rather than pre-mixing in a tank. This can work, but there's a catch: there's always a delay between when chemical enters the line and when it reaches the return conductivity sensor.
That delay creates overshoot. The system keeps dosing because it hasn't "seen" the chemical yet, and by the time the sensor reads the increase, you've already added too much. The result is chemical waste. With chemical costs rising, waste adds up fast.
Facilities using inline dosing should watch for this pattern in their data: conductivity spiking well above setpoint before settling back down. That spike is money going down the drain.
Clean-in-Place Steps and Procedures
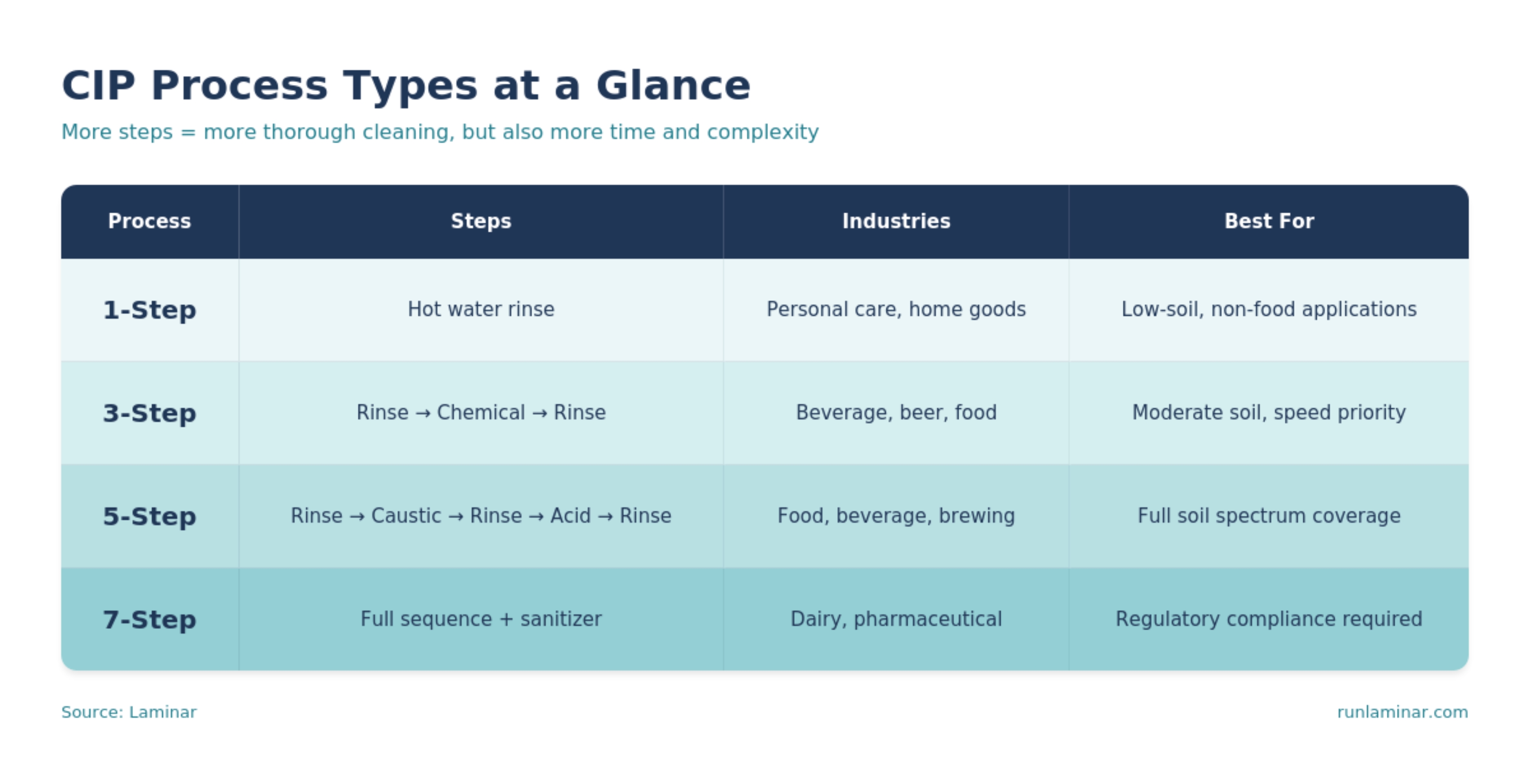
CIP Process Types: 1-Step, 3-Step, 5-Step, and 7-Step
There are multiple types of CIP programs with varying CIP cleaning steps that each have unique functions.
The choice between a 3-step, 5-step, or 7-step process comes down to three factors: what you're cleaning, what industry you're in, and how much risk you're willing to accept. More steps means more thorough cleaning—but also more complexity, more variables, and more time offline. Understanding these tradeoffs is where optimization begins.
CIP cleaning processes are made up of a few standard steps that are run at various lengths and intensities. The CIP steps include some variation of the following components:
- Pre-Rinse Water Rinse: This is water used to rinse out major contaminants and soils in the line or equipment. This can be fresh water or reclaimed water (usually used final rinse water)
- Caustic: Caustic solution heated to 80C to remove fats, proteins, and hard to clean materials
- Intermediate Rinse: Water used to remove chemicals after a chemical step such as a caustic or acid step before the beginning of another chemical step in the CIP.
- Acid: Acid solution used to remove scale, minerals, and soils in the line.
- Sanitation Step: This is used to disinfect the line to kill microbes. This is often a PAA solution or hot water rinse.
- Final Rinse: Water used to remove chemicals before the end of the CIP.
A CIP will always include a hot flush or chemical flushin one of the steps. Cold water flush is usually classified as a changeover. Most CIP cycles include a chemical wash, however, a hot rinse only CIP can sometimes be found in personal care or home good production lines that may not have the same hygiene requirements found in food and beverage regulations.
Here's a high-level view of the type of CIP programs:
1 Step Clean-in-Place Process
A 1-Step CIP Process is typically used to disinfect and kill bacteria with hot water. If only cold water is used, it's typically classified as a changeover — not a true CIP.
What industries typically use 1-Step CIP Process? Personal Care or Home Products that don't require a higher level of hygiene like food and beverage.
Step 1: Rinse with a hot water flush.
3 Step Clean-in-Place Process
A 3-Step CIP Process is typically used to clean materials hard to remove with only water. It introduces a chemical step such as caustic or acid that increases cleaning strength.
What industries typically use 3-Step CIP Process? Beer, Food, Beverage, Flavors, Fragrance.
- Step 1: Rinse with water
- Step 2: Rinse with a chemical (acid or caustic)
- Step 3: Final rinse with fresh water
Why choose 3-step CIP process? It's the simplest chemical CIP and the fastest to run. Facilities choose 3-step when soil loads are moderate, product compatibility is high, and speed matters. It's common in beverage operations with frequent changeovers between similar products—think regular cola to diet cola, or one juice flavor to another.
The tradeoff is that you're relying on a single chemical to do all the heavy lifting, which may not always work depending on the product needed to be cleaned out. If you're seeing consistent validation failures or residue complaints with a 3-step CIP, moving to a 5-step CIP or extending cycle times should be considered.
5 Step Clean-in-Place Process
A 5-Step CIP Process is a much stronger CIP process reserved for dirty-to-clean pieces of equipment or a dirty-to-clean line that has been running for a while. 5 Step CIP processes introduce second chemical step to increase cleaning action.
What industries typically use 5-Step CIP Process? Beer, Food, Beverage, Flavors, Fragrance, and Pharmaceuticals.
- Step 1: Rinse with water
- Step 2: Rinse with a chemical (acid or caustic)
- Step 3: Intermediate rinse with water
- Step 4: Rinse with a chemical (This may be acid; however, a sanitizer may be introduced in this step too)
- Step 5: Final rinse with fresh water flush
Why choose a 5-step CIP process? Five-step is where most food and beverage facilities land. You get caustic for organics and acid for mineral scale, which covers the full range of soils. The intermediate rinse between chemicals is critical—caustic and acid can't mix, and residual caustic will neutralize your acid step if it's not rinsed.
This is also where complexity starts to matter. Five steps means five opportunities for something to go wrong: a step that advances too early, a chemical that doesn't reach setpoint, a rinse that doesn't fully clear. Facilities running 5-step CIPs typically have a lot of room for optimization — there are simply more variables to tune.
7 Step CIP Process
A 7-Step CIP Process a long CIP process used where sanitation is critical.
What industries typically use 7-Step CIP Process? Beer, Food, Beverage, and Pharmaceuticals.
- Step 1: Rinse with water
- Step 2: Rinse with a chemical (acid or caustic)
- Step 3: Intermediate rinse with water
- Step 4: Rinse with a chemical (acid, caustic, or sanitizer)
- Step 5: Intermediate rinse with water
- Step 6: Rinse with a sanitizer
- Step 7: Final rinse
Why choose a 7-Step CIP Process? In dairy and pharma, 7-step isn't really a choice—it's practically mandated. Regulatory requirements and the consequences of contamination (product recalls, facility shutdowns) make the longer cycle non-negotiable. The dedicated sanitizer detergent step provides documented microbial kill that auditors and quality teams require.
But here's the tradeoff: 7-step CIPs have the most variables, the longest cycle times, and the highest resource consumption. With high complexity, they also have the most room for error — and the most room for savings. When every CIP has seven steps running multiple times per day, even small per-step optimizations compound quickly. A facility running 10 CIPs per day that saves 2 minutes per step across 7 steps reclaims over 2 hours of production time daily.
Choosing the Right CIP Process: Risk vs. Efficiency
The jump from 3-step to 5-step to 7-step isn't just about cleaning power—it's about risk tolerance.
A 3-step CIP is a bet that your soils are manageable and your products are compatible. It's the right call for many beverage and food applications, and it keeps lines running. A 7-step CIP is a bet that the cost of contamination far exceeds the cost of downtime. In dairy and pharma, that math is obvious. In other industries, it's a judgment call.
The problem is that many facilities aren't making that judgment call with data. They're running the CIP program that was set up a decade ago, designed for worst-case conditions that may or may not reflect what's actually happening on the line today. The result: 7-step CIPs running where 5-step would suffice, or 3-step CIPs struggling with soils they weren't designed for.
Understanding which process fits your actual conditions—not your assumed conditions—is the first step toward optimization.
Why Run a Caustic Step before an Acid Step?
The difference between a 3 Step CIP and a 5 Step CIP process is the need to run both a caustic and an acid step. In a five step CIP process, the first chemical rinse should be a caustic step, and the second will be acid.
Caustic has a very high pH and can result in calcium carbonate buildup and scale on the pipes when running for a long time. The acid wash serves a dual purpose to both clean the piece of equipment and remove the scale from the lines.

CIP System Equipment and System Design
How CIP Systems Clean Different Equipment
CIP systems are designed to clean equipment that can't be easily disassembled—or where disassembly would create unacceptable downtime. The cleaning approach varies based on equipment geometry, soil type, and how solution flows through the system.
Tanks, Blenders, and Vessels
Tanks, Blenders, and Vessels Tanks are cleaned using spray balls or rotating spray devices mounted inside the vessel. CIP steps often include multiple rinse and drain pulses—pulsing is more effective than continuous flow because it creates turbulence and reaches surfaces that a steady stream would miss. Tanks cannot be cleaned with pigs due to their geometry.
Piping
Piping is the simplest equipment to clean via CIP. Continuous flow pushes solution through the line, and the pipe's narrow diameter creates the velocity needed for effective cleaning. Pigs (pipeline inspection gauges—flexible plugs pushed through the line) can be used before CIP to clear product and reduce soil load, minimizing water and chemical use during the actual cleaning cycle.
Valves, Hoses, and Spray Devices
Valves, flexible hoses and spray assemblies require dedicated attention because their geometry can trap product and resist flow. Valves and hoses are typically cleaned inline with the piping they connect to, but facilities should verify that flow rate through hoses is sufficient—a valve disturbing flor or a hose with a larger diameter than the connected pipe may not achieve adequate velocity. Spray balls and rotating spray heads also need periodic COP cleaning to clear clogged nozzles, which can create shadow zones inside tanks if left unaddressed.
Filling Stations
Filling stations are the most complex CIP targets. A single filler may have dozens of valves, manifolds, and flow paths—each of which needs to be flushed during cleaning. CIP programs for fillers typically include multiple sub-steps that cycle through different flow paths within a single cleaning phase. If you're troubleshooting a filler CIP, confirm that all sub-steps are actually running; incomplete path coverage is a common cause of validation failures.
Heat Exchangers
Plate and tubular heat exchangers accumulate scale and burnt-on deposits, particularly in dairy and beverage applications where product is pasteurized or heated. CIP for heat exchangers typically requires extended caustic contact times and periodic acid cycles to remove mineral buildup. Flow direction may be reversed during CIP to clean surfaces that don't see adequate flow in normal operation.

What is a CIP Skid?
A CIP skid (also called a CIP station or CIP system) is a self-contained unit that stores, heats, and pumps cleaning solutions through processing equipment. It includes tanks, pumps, heat exchangers, and instrumentation to automate the cleaning cycle.
Core CIP Skid components include:
- Tanks for water and chemical solution storage
- Pumps to circulate solution through equipment
- Heat exchangers to bring solution to target temperature
- Piping and valves to control flow and deliver solution to equipment
- Instrumentation to measure temperature, conductivity, and flow rate
The specific CIP equipment configuration depends on facility size, the number of lines being cleaned, and whether chemicals are single-use or reclaimed. But every CIP machine, from a compact single-tank skid to a multi-tank, multi-loop central system, includes these core components.
Clean-in-Place System Types:
Single Use vs. Reuse
CIP systems fall into two categories based on how they handle chemicals: Single-Use (Type I) or Reuse or recirculating (Type II). The distinction affects capital cost, operating cost, and where optimization opportunities live.
Single Use CIP Skid (Type 1)
In a Single Use (Type 1) system, chemical solution is prepared fresh for each CIP cycle. Once cleaning is complete, the spent solution is discharged to drain. Nothing is reclaimed or reused.
Type I skids are usually smaller with lower upfront capital investment. They show up more often in smaller operations, and older plants. Some Single-Use Skids are designed to be mobile. The trade-off is chemical usage: every CIP requires remaking the solution from scratch, which adds up fast.
How to identify a Type I system: If the CIP skid has only one tank, it's always Type I. There's no way to reclaim chemicals without dedicated storage.
Reuse CIP Skid (Type II)
In a Reuse system (Type II), the chemical solution is stored in a central holding tank and recirculated across multiple cleaning cycles. As the solution becomes diluted or loses strength, it gets re-dosed to restore concentration. If the tank volume drops below a certain level, fresh chemical solution is created and added.
Type II skids are larger and require multiple tanks — dedicated chemical storage plus a water tank. The upfront investment is higher, but chemical usage is significantly lower since the same solution is reclaimed and reused across subsequent CIPs.
Type II systems are more common in newer, larger facilities where the CAPEX pays off in long-term chemical efficiency.
Single-loop vs. Multi-loop CIP Systems
A CIP station can have a single loop — one supply line and one return line — or multiple loops, with several supply and return lines going out to different pieces of equipment.
The components are the same either way: tanks for water and chemicals, heat exchangers, and pumps. The difference is scale and how those resources are shared.
Multi-loop CIP stations share a central chemical and water tank across all their loops, which means multiple pieces of equipment can be cleaned at the same time. Multi-loop systems are almost always Type II CIPs.
Single-loop stations are usually smaller with lower CAPEX. These show up more often in smaller operations, and older facilities. Type II multi-loop stations tend to appear in newer, larger facilities where the upfront investment pays off in chemical efficiency.
Keep reading for a look at our Clean-in-Place is used in different industries.

Clean-in-Place Industry Spotlights
Industry Spotlight: CIP in Food Processing
Food processing is interesting because you've got such a wide variety — sauces, soups, ready meals, condiments. And the frequency really depends on what you're running. Sauces, for example, can go multiple days to a week between sanitations. But the thing is, when you're changing from one product to another and those products aren't compatible with each other, you can't just do a product push or a water flush. You need a full CIP.
At a Glance
- Frequency: Multiple days to a week, depending on product type
- Primary soils: Heavy loads—starches, proteins, fats
- Typical programs: 5-step common; extended caustic contact for stubborn soils
- Critical concern: Allergen cross-contamination between incompatible products
Industry Spotlight: CIP in Beverage
Beverage manufacturers runs CIP every one to a few days — more frequently than food processing, but less than dairy. The challenges are sugar residue and flavor carryover, especially in times of SKU proliferation or during high-changeover periods with seasonal or promotional SKUs. Three-step CIPs are standard; five-step for stickier products.
At a Glance
- Frequency: Every 1-3 days; increases during high-changeover periods
- Primary soils: Sugar residue, flavor compounds
- Typical programs: 3-step standard (rinse, chemical, final rinse); 5-step for sticky products
- Watch metric: Flavor carryover complaints; filling line availability
Industry Spotlight: CIP in Brewing
Breweries face challenges in beverage facilities with high SKUs but their product also have proteins and yeast. Microbe contamination is critical to prevent to insure no product spoilage and improper fermentation. Breweries also face a unique challenge: scale buildup. Caustic has very high pH and causes calcium carbonate to accumulate on pipes over time. This is why five-step CIPs run caustic first, then acid. The acid does double duty, cleaning the equipment and removing the scale buildup that occurs from running caustic CIP repeatedly.
At a Glance
- Primary soils: Yeast residue, protein deposits, calcium carbonate scale (beerstone)
- Equipment complexity: Fermentation vessels, brite beer tanks, heat exchangers, filtration vessels, mashing tanks
- Typical programs: Caustic CIP between batches; periodic acid CIP for brite beer tankes
- Watch metric: Scale accumulation; tank turnaround time
Industry Spotlight: CIP in Flavors, Fragrances, and Dyes
Flavors, fragrances, and dyes are designed to be potent—which means even trace residue can contaminate the next batch. Most facilities run daily CIPs or multiple times per day. Time pressure is intense—though some run less frequently with much longer cycles, sometimes five to seven hours.
The bigger challenge is recipe design. These facilities often produce a different product every day, making it nearly impossible to design CIP recipes based on historical patterns or worst-case scenarios. What worked for yesterday's citrus fragrance may overwash or underwash today's vanilla.
At a Glance
- Frequency: Daily in high-changeover environments; some facilities run weekly with extended cycles (5-7 hours)
- Primary soils: Concentrated pigments, aromatic compounds, oils, resins
- Typical programs: 5-step or 7-step; recipe accuracy is a persistent challenge due to product variability
- Watch metric: CIP duration vs. production time; batch rejection rates
Industry Spotlight: CIP in Personal Care & Home Care
Health, beauty, personal care and home care manufacturing facilities have different requirements than food and beverage— the hygiene standards aren't as stringent. This is why 1-step CIPs are most common here: a hot water flush to disinfect and kill bacteria, without the full chemical cycles you see in food production. If it's just cold water, it's typically classified as a changeover, not a true CIP.
At a Glance
- Frequency: Varies widely; often at product changeover rather than timed intervals
- Primary soils: Oils, surfactants, fragrances, colorants, viscous bases
- Typical programs: 1-step (hot water rinse) most common; chemical CIP less frequent than food/bev
- Key difference: Lower hygiene requirements compared to food and beverage—less need for multi-step chemical cycles
- Watch metric: SKU changeover efficiency; dead leg contamination
Industry Spotlight: CIP in Dairy
Dairy facilities run CIP every 24-48 hours—among the most frequent in food manufacturing. Sanitation requirements are higher here, which is why 7-step CIPs are common: rinse, caustic, intermediate rinse, acid, intermediate rinse, sanitizer, final rinse. Both caustic and acid are required because milk proteins and mineral deposits demand different chemistry.
At a Glance
- Frequency: Every 24-48 hours
- Primary soils: Milk proteins, fats, heat-set soils from pasteurization
- Typical programs: 5-step or 7-step; caustic + acid required
- Watch metric: CIP downtime vs. milk receiving capacity
Industry Spotlight: CIP in Pharmaceuticals
In pharma, documentation is as critical as cleaning. FDA/cGMP requirements mean every cycle must be validated, traceable, and audit-ready. Cross-contamination between APIs is the primary concern.
At a Glance
- Regulatory context: FDA/cGMP; cleaning validation protocols required
- Critical concern: API cross-contamination
- Key difference: Traceability carries equal weight to cleaning efficacy
- Watch metric: Validation success rate; audit readiness
Industry Spotlight: CIP in Oil and Gas
Oil and gas CIP differs significantly from food and beverage applications. The goal isn't sanitation—it's maintaining flow, preventing corrosion, and removing deposits that reduce equipment efficiency.
Pipeline cleaning in oil and gas often relies on pigging (mechanical cleaning) rather than chemical CIP, but tanks, heat exchangers, and process vessels do undergo in-place cleaning. The chemistry is different too: hydrocarbon solvents and specialized surfactants replace the caustic/acid/sanitizer sequence used in food manufacturing.
At a Glance
- Primary soils: Hydrocarbons, paraffin wax, asphaltenes, mineral scale, sand, corrosion products
- Typical programs: Solvent flush, surfactant wash, water rinse; mechanical pigging common for pipelines
- Key difference: Sanitation not required; focus is on flow assurance and corrosion prevention
- Watch metric: Flow rate recovery; heat exchanger efficiency; corrosion monitoring
.jpg)
Frequently Asked Questions:
Clean-in-Place
What does CIP stand for in manufacturing?
CIP stands for clean-in-place. It refers to the automated cleaning of processing equipment—pipes, tanks, vessels, and filling lines—without disassembly. CIP is the standard cleaning method in food and beverage, dairy, pharmaceutical, and personal care manufacturing.
What is the difference between CIP and COP?
CIP (clean-in-place) cleans equipment without taking it apart, using circulated water and chemicals. COP (clean-out-of-place) requires disassembling components and cleaning them manually or in a separate washing unit. Most facilities use both: CIP for tanks and piping, COP for fittings, gaskets, and parts that trap residue.
How long does a CIP cycle take?
CIP cycle duration typically ranges from 30 minutes to 2 hours for standard 3-step and 5-step processes. Seven-step CIPs in dairy and pharmaceutical facilities usually run 60-90 minutes. Extended CIPs in industries like flavors and fragrances can take 5-7 hours when cleaning potent residues.
What chemicals are used in CIP cleaning?
The three main CIP chemicals are caustic (sodium hydroxide), acid (nitric, phosphoric, or citric), and sanitizer (peracetic acid or PAA). Caustic removes organic soils like fats and proteins. Acid removes mineral scale and deposits. Sanitizer provides a final antimicrobial kill step.
How often should CIP be performed?
CIP frequency depends on the industry and product type. Dairy facilities typically run CIP every 24-48 hours. Beverage manufacturers clean every 1-3 days. Food processing facilities may go multiple days to a week between CIPs. CIP is also triggered when switching between incompatible products.
What is a CIP skid?
A CIP skid is a self-contained cleaning unit that stores, heats, and pumps cleaning solutions through processing equipment. Core components include tanks for water and chemicals, pumps, heat exchangers, and instrumentation to monitor temperature, conductivity, and flow. CIP skids are either Type I (single-use chemicals) or Type II (recirculating chemicals).
Laminar Clean-in-Place Optimization
Thanks for reading!
Check out our blog for more resources on Clean-in-Place. Interested in how Laminar helps liquid manufacturers optimize clean-in-place? Check out the link below.
Related Blog Posts

From 300 to 3,300 SKUs: When Clean-in-Place Becomes the Bottleneck




.svg)

